

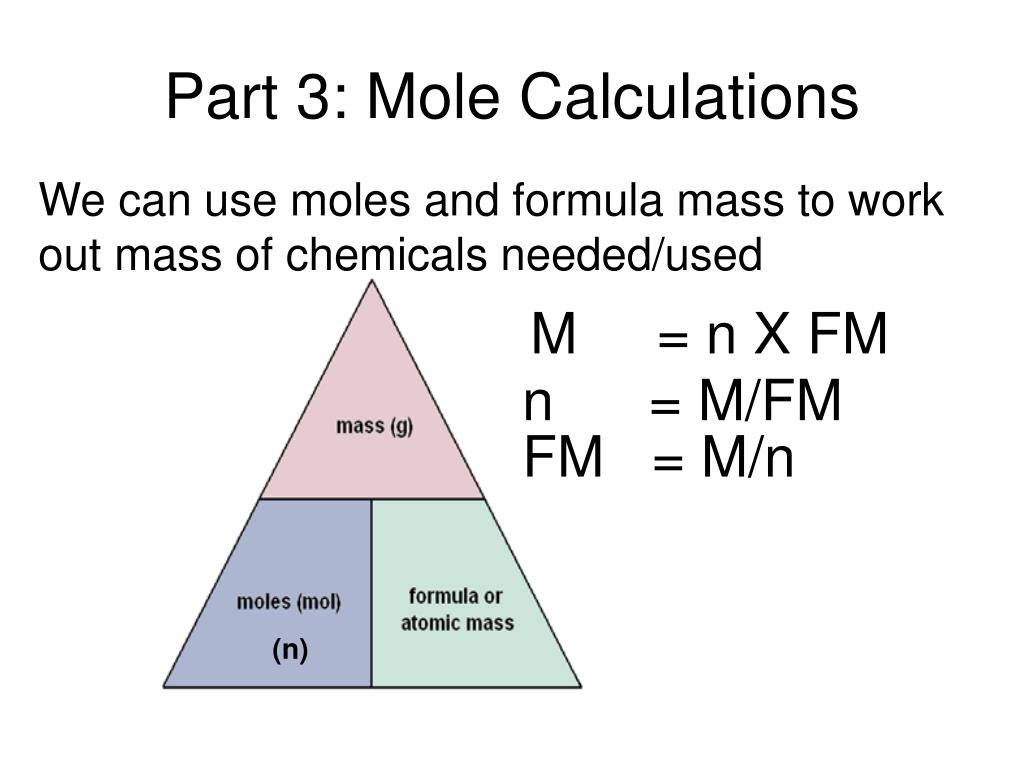

This is a useful tool which allows you to calculate the: Remember that one cubic decimeter equals to one liter, so these two notations express the same numeric values. In many older books or articles, you can find different units of molar solutions - moles per liter (mol/l). The molar concentration of solute is sometimes abbreviated by putting square brackets around the chemical formula of the solute, e.g., the concentration of hydroxide anions can be written as. They are noted as mol/dm³ as well as M (pronounced "molar"). The units of molar concentration are moles per cubic decimeter. If the amount of solute is given in grams, we must first calculate the number of moles of solute using the solute’s molar mass, then calculate the molarity using the number of moles and total volume. To calculate the molarity of a solution, the number of moles of solute must be divided by the total liters of solution produced. The molarity could be calculated by dividing one mole by this total volume. After the solution has been mixed, the volume would be recorded. For example, if one mole of salt were added to one liter of water, the resulting solution would not be 1 M (read as "one molar"). It is important to note that molarity is per liters of solution, not per liters of solvent. Molarity is defined as moles of solute per liters of solution. The standard unit of concentration in chemistry is molarity (abbreviated with M). There are many different units of concentration. The concentration of the solution tells you how much solute has been dissolved in the solvent. In vinegar, acetic acid is the solute and water is the solvent and in bleach, sodium hypochlorite is the solute and water is the solvent. So in the salt water example, the salt is the solute and the water is the solvent. The solute is the material that is dissolved while the solvent is whatever it is dissolved in. All of these examples have both a solute and a solvent. Similarly, bleach is a solution of sodium hypochlorite. If you look closely at a bottle of vinegar, you will find that it is a solution of acetic acid. Most of your "household" chemicals are solutions. The salt dissolves in the water, resulting in a solution. For example, you may add salt to water when cooking pasta. In your everyday life, you encounter solutions all the time. Mass (g) = Concentration (mol/L) x Volume (L) x Molecular Weight (g/mol) The molarity calculator is based on the following equation:


 0 kommentar(er)
0 kommentar(er)
